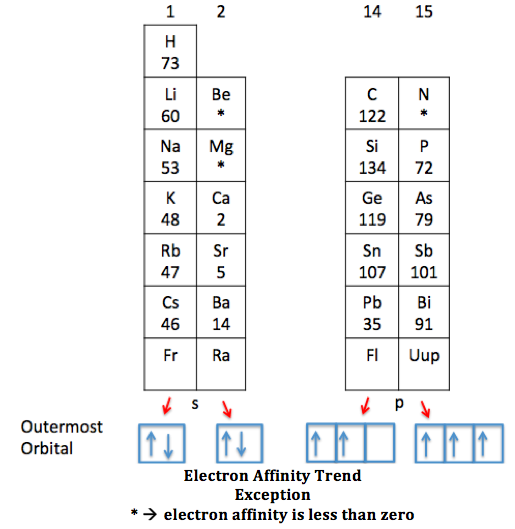
Why Does Chlorine Have Higher Electron Affinity Than Fluorine . Since this periodic property tends from the bottom. Web and yes, chlorine has a higher electron affinity than fluorine. Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. That is because although fluorine wants to attract. Web the larger pull from the closer fluorine nucleus is why fluorine is more electronegative than chlorine is. Summarising the trend down the group. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. Consequently, the elements of the third. Web fluorine, therefore, has a lower affinity for an added electron than does chlorine. Web why does chlorine have a higher electron affinity value than fluorine?
from study.com
Since this periodic property tends from the bottom. Consequently, the elements of the third. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. Summarising the trend down the group. Web why does chlorine have a higher electron affinity value than fluorine? Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. Web fluorine, therefore, has a lower affinity for an added electron than does chlorine. Web the larger pull from the closer fluorine nucleus is why fluorine is more electronegative than chlorine is. Web and yes, chlorine has a higher electron affinity than fluorine. That is because although fluorine wants to attract.
Electron Affinity Definition, Trends & Equation Video & Lesson
Why Does Chlorine Have Higher Electron Affinity Than Fluorine Consequently, the elements of the third. Since this periodic property tends from the bottom. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. That is because although fluorine wants to attract. Consequently, the elements of the third. Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. Web why does chlorine have a higher electron affinity value than fluorine? Web the larger pull from the closer fluorine nucleus is why fluorine is more electronegative than chlorine is. Summarising the trend down the group. Web fluorine, therefore, has a lower affinity for an added electron than does chlorine. Web and yes, chlorine has a higher electron affinity than fluorine.
From material-properties.org
Fluorine and Chlorine Comparison Properties Material Properties Why Does Chlorine Have Higher Electron Affinity Than Fluorine Web why does chlorine have a higher electron affinity value than fluorine? Summarising the trend down the group. Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. Consequently, the elements of the third. That is because although fluorine wants to attract. Web fluorine, therefore, has a lower affinity for an. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.nagwa.com
Question Video Identifying the Explanation for the Anomalous Electron Why Does Chlorine Have Higher Electron Affinity Than Fluorine Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. Web the larger pull from the closer fluorine nucleus is why fluorine is more electronegative than chlorine is. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. Since this periodic property tends from the bottom. That. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.youtube.com
Why Electron Affinity of Fluorine is lower than Chlorine by Tariq Why Does Chlorine Have Higher Electron Affinity Than Fluorine That is because although fluorine wants to attract. Web why does chlorine have a higher electron affinity value than fluorine? Fluorine, though higher than chlorine in the periodic table, has a very small atomic. Consequently, the elements of the third. Summarising the trend down the group. Web fluorine, therefore, has a lower affinity for an added electron than does chlorine.. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.toppr.com
The electron affinity of fluorine is less than that of chlorine because Why Does Chlorine Have Higher Electron Affinity Than Fluorine Web the larger pull from the closer fluorine nucleus is why fluorine is more electronegative than chlorine is. Web why does chlorine have a higher electron affinity value than fluorine? Fluorine, though higher than chlorine in the periodic table, has a very small atomic. That is because although fluorine wants to attract. Web fluorine, therefore, has a lower affinity for. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.numerade.com
SOLVEDUse electron configurations to explain why (a) sulfur has a Why Does Chlorine Have Higher Electron Affinity Than Fluorine Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. Web fluorine, therefore, has a lower affinity for an added electron than does chlorine. That is because although fluorine wants to attract. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. Web why does chlorine have. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From sciencenotes.org
Electron Affinity Trend and Definition Why Does Chlorine Have Higher Electron Affinity Than Fluorine Web and yes, chlorine has a higher electron affinity than fluorine. Summarising the trend down the group. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. That is because although fluorine wants to attract. Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. Consequently, the. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From mungfali.com
Fluorine Electron Configuration Diagram Why Does Chlorine Have Higher Electron Affinity Than Fluorine That is because although fluorine wants to attract. Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. Web the larger pull from the closer fluorine nucleus is why fluorine is more electronegative than chlorine is. Web fluorine, therefore, has a lower affinity for an added electron than does chlorine. Fluorine,. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.youtube.com
Statement1 Fluorine has less electron affinity than chlorine Why Does Chlorine Have Higher Electron Affinity Than Fluorine Fluorine, though higher than chlorine in the periodic table, has a very small atomic. That is because although fluorine wants to attract. Web and yes, chlorine has a higher electron affinity than fluorine. Web fluorine, therefore, has a lower affinity for an added electron than does chlorine. Since this periodic property tends from the bottom. Summarising the trend down the. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.toppr.com
Although electron gain enthalpy of fluorine is less negative as Why Does Chlorine Have Higher Electron Affinity Than Fluorine Web and yes, chlorine has a higher electron affinity than fluorine. That is because although fluorine wants to attract. Consequently, the elements of the third. Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. Web why. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.youtube.com
The lower electron affinity of fluorine than that of chlorine is due to Why Does Chlorine Have Higher Electron Affinity Than Fluorine Since this periodic property tends from the bottom. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. Web and yes, chlorine has a higher electron affinity than fluorine. Web why does chlorine have a higher electron affinity value than fluorine? That is because although fluorine wants to attract. Web the larger pull from the closer. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Why Does Chlorine Have Higher Electron Affinity Than Fluorine Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. Summarising the trend down the group. Web why does chlorine have a higher electron affinity value than fluorine? Consequently, the elements of the third. Since this periodic property tends from the bottom. Fluorine, though higher than chlorine in the periodic table,. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.nagwa.com
Question Video Recalling the Species Formed When a Chlorine Atom Gains Why Does Chlorine Have Higher Electron Affinity Than Fluorine Since this periodic property tends from the bottom. Web why does chlorine have a higher electron affinity value than fluorine? Consequently, the elements of the third. Web fluorine, therefore, has a lower affinity for an added electron than does chlorine. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. Web and yes, chlorine has a. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.nagwa.com
Question Video Determining the Equation that Represents the Electron Why Does Chlorine Have Higher Electron Affinity Than Fluorine Web why does chlorine have a higher electron affinity value than fluorine? Web and yes, chlorine has a higher electron affinity than fluorine. Summarising the trend down the group. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. That is because although fluorine wants to attract. Web fluorine, therefore, has a lower affinity for an. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.youtube.com
Chlorine has ___________ electron affinity than fluorine. YouTube Why Does Chlorine Have Higher Electron Affinity Than Fluorine Consequently, the elements of the third. Since this periodic property tends from the bottom. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. Web and yes, chlorine has a higher electron affinity than fluorine. Web why does chlorine have a higher electron affinity value than fluorine? Summarising the trend down the group. Web the larger. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From ecurrencythailand.com
What Is The Electron Affinity Of Fluorine? All Answers Why Does Chlorine Have Higher Electron Affinity Than Fluorine Web fluorine, therefore, has a lower affinity for an added electron than does chlorine. Web and yes, chlorine has a higher electron affinity than fluorine. Consequently, the elements of the third. That is because although fluorine wants to attract. Since this periodic property tends from the bottom. Summarising the trend down the group. Web the larger pull from the closer. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Why Does Chlorine Have Higher Electron Affinity Than Fluorine Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. Web why does chlorine have a higher electron affinity value than fluorine? Web fluorine, therefore, has a lower affinity for an added electron than does chlorine. Web the larger pull from the closer fluorine nucleus is why fluorine is more electronegative. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Why Does Chlorine Have Higher Electron Affinity Than Fluorine Consequently, the elements of the third. Fluorine, though higher than chlorine in the periodic table, has a very small atomic. Since this periodic property tends from the bottom. That is because although fluorine wants to attract. Web the reason why chlorine has a higher electron affinity than fluorine is because the fluorine atom is smaller. Summarising the trend down the. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.
From study.com
Electron Affinity Definition, Trends & Equation Video & Lesson Why Does Chlorine Have Higher Electron Affinity Than Fluorine Web the larger pull from the closer fluorine nucleus is why fluorine is more electronegative than chlorine is. Consequently, the elements of the third. Web and yes, chlorine has a higher electron affinity than fluorine. Web fluorine, therefore, has a lower affinity for an added electron than does chlorine. Web why does chlorine have a higher electron affinity value than. Why Does Chlorine Have Higher Electron Affinity Than Fluorine.